BEHIND THE SCENES IN THE IVF LAB: From Egg Retrieval to Embryo Transfer
As a fertility doctor, I know the ins and outs of the IVF lab and all of the procedures that take place. As a patient who underwent IVF to preserve my fertility via embryo freezing, I had the major advantage of feeling completely at ease with the process because I knew exactly what to expect and everything that was happening with our eggs, sperm, and embryos at every step of the way. Knowing the comfort that knowledge brought me, makes me want to explain the entire process and everything that happens behind the scenes to every single patient, in an effort to make them feel just as comfortable and confident in the process as I did.
This is why I put together this overview of the steps taken in the IVF lab to get from egg to embryo.
Egg
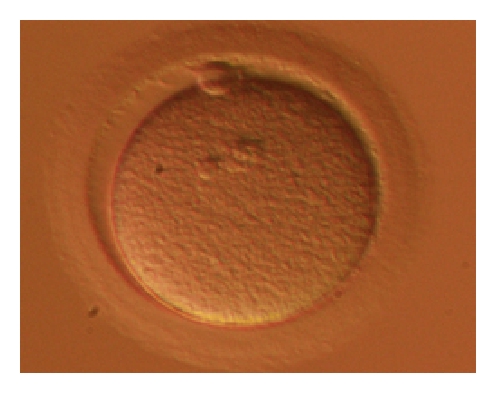
During the IVF process, eggs are retrieved using a long, thin needle which passes through the wall of the vaginal canal to enter the adjacent ovary and drain each follicle (or bubble of fluid) which contains an egg. By draining the fluid, eggs are picked up from each follicle in both ovaries. The fluid is passed to an embryologist, who is a highly specialized technician that is trained to handle microscopic cells such as egg, sperm, and embryos and to perform all the necessary procedures in the IVF lab. The eggs are then counted under a microscope. At this point they can be stripped of the surrounding cells and frozen (if the plan is egg freezing).
Fertilized Egg
If the plan is in vitro fertilization, the eggs can either be incubated with ~50,000 sperm each and then left to interact with the sperm and be fertilized the ‘conventional’ way… OR the eggs can be stripped of the surrounding cells, assessed for maturity (there are 3 major stages of egg development – which can only be discerned if the egg is denuded of its surrounding ‘support’ cells), and injected with a single sperm cells (‘Intracytoplasmic Sperm Injection’ or ICSI). ICSI is used for a variety of reasons, including cases where there is a major sperm quality issue (ie. low concentration/motility), if eggs were previously frozen, and in many cases when genetic testing of embryos is planned (to avoid DNA contamination of the embryo genetic testing sample with extra sperm that didn’t actually fertilize the egg).
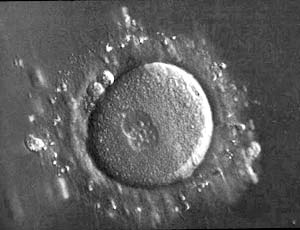
About 18 hours after the eggs are inseminated, they can be assessed to see whether they fertilized successfully. This is signified by the appearance of specific components: 2 different ‘nuclei’ and 2 ‘polar bodies’ which appear as 2 small cells sequestered to the periphery of the egg. Only the fertilized eggs that have this appearance can continue on in the IVF lab to grow into embryos over the next 3-7 days. In general, ~70-80% of eggs fertilize successfully – however, this will depend on individual factors such as sperm and egg quality.
Day 3 (or ‘Cleavage Stage’) Embryo
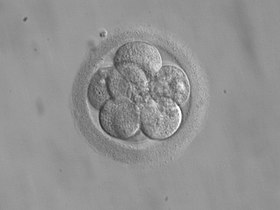
Successfully fertilized eggs can be further incubated and grown into embryos. The incubators used in the IVF lab have very specific temperature and gas settings. They must be maintained at body temperature (~37 degrees Celsius) and have a low oxygen environment (5% oxygen) to mimic the typical environment (the reproductive tract) where the embryo would form on its own inside the body. Additionally, the embryologists will incubate the embryos in fluid, or ‘media’, which is supplemented with nutrients and other substances that provide the embryo with the essential energy and support needed to grow and advance through the various stages of development. The embryos may be checked on again after fertilization by day 3 post-fertilization. At this stage, they typically have grown and divided into anywhere from 6-10 cells. It used to be commonplace to transfer embryos at this stage of development back into the uterus. Some IVF clinics may still opt to perform day 3 embryo transfers. Getting to day 3 is not deemed a huge challenge – most fertilized eggs can grow to day 3 and many of us fertility doctors regard embryos prior to day 3 as being on a kind of ‘autopilot’ where they are just going through the motions and following the instructions for growth and development inherited from the sperm and egg. It doesn’t take much to get to day 3 and reaching this stage does little to reassure us about the quality of an embryo- the real challenge lies beyond this time point.
Day 4 – Compacted Embryo or Morula Stage Embryo
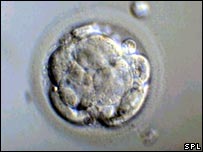
The 6 to 10 separate cell of the day 3 embryo begin to coalesce, with cell borders becoming indistinguishable. The once-distinct cells now appear compacted into a single mass. This is the transition stage between a day 3 or cleavage stage embryo and a blastocyst. This is a highly dynamic and short stage. Most IVF labs will try and minimize the number of times that embryos are removed from the incubator – so it is highly possible the embryos will not be viewed or checked on at this stage.
Day 5 to 7 – Blastocyst Stage Embryo
Embryos that successfully develop into day 3 embryos may be further incubated so that they can grow to the blastocyst stage (day 5 to 7 post-fertilization). Typically, this involves switching the embryos to an environment, or media, that contains more glucose to support the high energy demands beyond day 3 of development. It is a widely accepted notion that it is at day 3 that the embryo starts to extinguish the instructions/programs inherited from the sperm and egg and begins to express its own genes – this is a biologically complex and demanding process and often does not happen as it should. This is thought to be a large part of why only ~60% of fertilized eggs make it to the blastocyst (day 5-7) stage. This is normal and something to be mentally prepared for if you are going through IVF treatment. There is a high rate of attrition going from fertilized egg to day 5 embryo and beyond and IT’S OKAY.
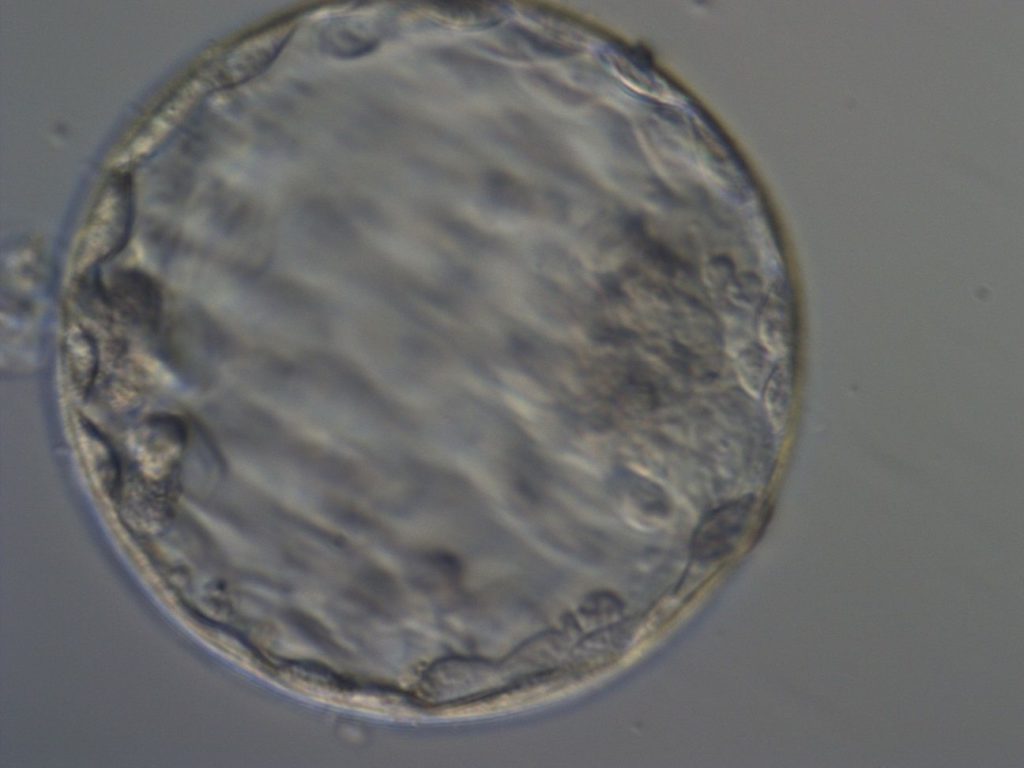
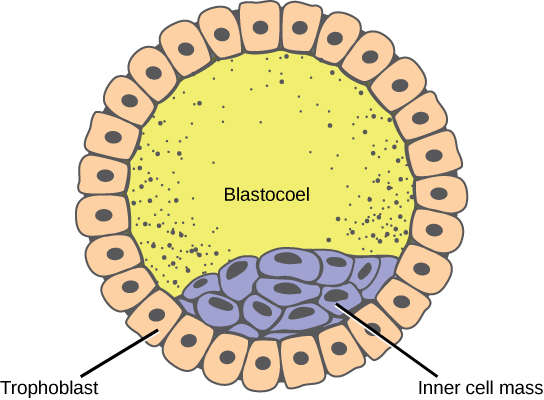
Getting to the blastocyst stage means that the embryo has divided into 100-200 cells which have clearly differentiated into two cells types – a central cluster of cells (‘inner cell mass’) will one day become the actual baby itself, and the cells that get sequestered out toward the periphery (‘trophectoderm’) will one day become the placenta.
Embryos at this stage of development can be genetically tested by biopsying the embryo, or removing a few of these ‘future placenta’ cells. If an embryo does not grow fast enough, with clear delineation between the two cell types by day 5, the embryo may be reincubated and then rechecked on day 6, or even day 7, with slower developing embryos being biopsied and frozen on these days, if they meet the criteria required to be safely tested and frozen. Embryos are then frozen by flash freezing – a process called ‘vitrification’, where the embryo is plunged into -196 degrees Celsius liquid nitrogen. Embryos can also be transferred into the uterus right away as a ‘fresh embryo transfer’, or frozen embryos can be thawed out and transferred to the uterus in a ‘frozen embryo transfer’ cycle. When vitrification is used, the embryo warming process is associated with very high survival rates – embryos at this stage of development tend to withstand the freeze-thaw process very well with rates of survival in the high 90s%.
Many IVF clinics will preferentially grow embryos to the blastocyst stage prior to transfer, to weed out the weaker, unhealthy embryos with low reproductive potential that stop growing in the lab on their own, as this is associated with higher implantation rates.
And that’s the basic overview of what happens in the IVF lab from egg retrieval to embryo transfer!
Please leave me any specific questions you may have about what happens in an IVF laboratory in the comments below!


